Domestic thermal analysis instrument professional manufacturer
Dedicated to thermal analysis instrument for more than 20 years
Thermal analysis of fireproof components of intumescent fireproof coatings

Yang Shousheng, Department of Fire Department, Armed Police College Pan Lu
1. Preface
As an important functional coating, the development of high quality fireproof coating is one of the hot research topics in the coating science. Expansive fireproof coating is a foam type, mainly composed of acid source, carbon source and blowing agent, etc., in case of fire or high temperature, under the synergistic action of the above three main components, the coating can rapidly expand dozens of times into a foamy carbonized layer, so as to effectively prevent the substrate from catching fire, and can be used in places with high fire protection grade.
Intumescent fire retardant coatings mainly function in the condensed phase. When the material is heated, the polymer flame retardant system forms a fluffy porous carbon layer, which plays the role of heat insulation and oxygen insulation. The amount of volatile combustible material produced during thermal degradation of polymer is reduced, and the purpose of flame retardation and smoke suppression is achieved. It is precisely because the generation of the expanded carbon layer overcomes the drip phenomenon of polymer combustion and stops the spread and spread of the flame, which gives the fire retardant coating a unique property superior to other fire retardant coatings.
Since 1990s, thermal analysis technology has been widely used in the evaluation of flammability and flame retardancy of materials. Thermal analysis can be divided into TGA, differential scanning calorimetry DSC, DTA and mechanical thermal analysis TMA and other methods. DSC can quantitatively analyze the thermal effects of polymer materials at different pyrolysis stages, which provides an effective means for evaluating the flame retardancy of materials and studying the flame retardancy mechanism. In this paper, differential scanning calorimeter was used to study the thermal properties of flame-retardant components in intumescent fire-retardant coatings, and its flame-retardant mechanism was preliminarily analyzed to provide theoretical basis for rational selection of flame-retardant formulation.
2. Experimental part
2.1 Test instrument
Differential scanning calorimeter: Swiss METTLE TA4000, DSC25, heating rate 10.0℃/min, air atmosphere, standard aluminum crucible, α-al2o3 as the reference, electronic analysis balance, DSC curve was processed by GraphWear TA72 software.
2.2 Experimental Drugs
(1) Carbonizing agent: pentaerythritol, starch, sugar
(2) Catalyst: ammonium polyphosphate, ammonium dihydrogen phosphate
(3) Blowing agent: melamine, dicyandiamide
(4) Sample 1: LF solvent steel structure expansion fireproof coating
(5) Sample 2: pentaerythritol + ammonium polyphosphate + melamine (mixing ratio 1:1:1)
(6) Sample 3: Starch + ammonium polyphosphate + dicyandiamide (mixing ratio 1:1:1)
3. Results and discussion
3.1 Thermal performance of a single component
The fire retardant mainly consists of three parts: char forming agent, catalyst and blowing agent. Therefore, the investigation of the thermal properties of a single component is the basis of studying the properties of flame retarders. There are many kinds of substances that can be used as fire retardants, and this paper only studies some of them which are widely used.
3.1.1 Carbonizing agent
Charcoal-forming agent is an important fireproof component of fireproof coatings, and its thermal decomposition behavior directly affects the fireproof performance of coatings. FIG. 1, 2 and 3 DSC curves of pentaerythritol, starch and sugar.
It can be seen from FIG. 1 that pentaerythritol has three endothermic peaks at 186℃, 241℃ and 345℃ respectively. The first endothermic peak is the dehydration peak, the second endothermic peak is the melting peak, and the third endothermic peak is the decomposition peak. This characteristic of gradient decomposition of heat absorption is of positive significance for the fire protection system of coatings.
As can be seen from Figure 2, starch has two endothermic peaks at 98℃ and 308℃ respectively, the first peak is the dehydration peak and the second peak is the decomposition peak, which produces a large amount of coal tar at the same time.
Figure 3 shows that sugar has two endothermic peaks at 189℃ and 229℃, the first endothermic peak is the melting peak and the second endothermic peak is the decomposition peak.
3.1.2 Catalyst
FIG. 4 and 5 DSC curves of ammonium polyphosphate and ammonium dihydrogen phosphate respectively. As can be seen from Figure 4, ammonium polyphosphate has two endothermic peaks at 123℃ and 185℃, and a continuous endothermic stage begins at about 290℃. The first endothermic peak is caused by dehydration, the second endothermic peak is the melting peak, and the continuous endothermic stage is the decomposition stage of ammonium polyphosphates, that is, from about 290 ammonium polyphosphates to decompose and play a catalytic role. As can be seen from Figure 5, ammonium dihydrogen phosphate has an endothermic peak at 207℃ and an exothermic peak at 216℃. The endothermic peak indicates that ammonium dihydrogen phosphate melts and decomposes at 207℃, and the exothermic peak may be caused by the oxidation of ammonia gas released by decomposition.
3.1.3 Blowing agent
FIG. 6 and 7 show the DSC curves of ammonium cyanamide and dicyandiamide respectively.
FIG. 6 shows that melamine only has an endothermic peak at 360 ° C, which indicates that ammonium cyanide decomposes and releases ammonia while sublimating at 360 ° C.
As can be seen from Figure 7, dicyandiamide has two endothermic peaks at 209℃ and 325℃, and an exothermic peak at 252℃. The first endothermic peak is the melting peak, the exothermic peak is caused by the release of heat when tautometry with melamine, and the second endothermic peak is its decomposition peak.
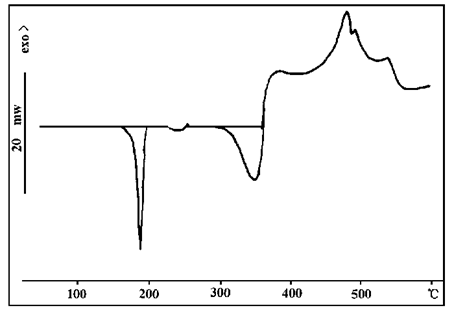
FIG. 1 DSC map of pentaerythritol
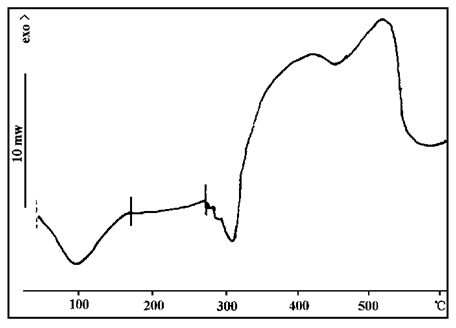
FIG. 2 DSC map of starch
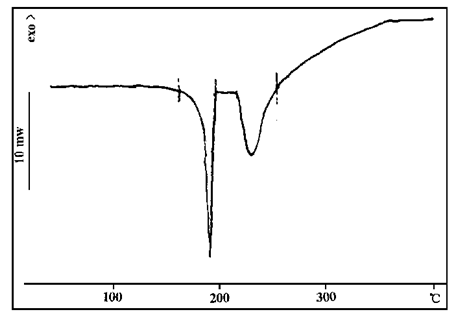
FIG. 3 DSC map of sugar
3.2 Thermal performance analysis of flame retardant system
3.2.1 Pyrolysis of flame retardant components after mixing
FIG. 9 and 10 show the DSC curves of sample 2.
Comparing Figure 9 and 10 with the thermal decomposition diagram of a single component above, it can be found that the decomposition behavior of each component changes after mixing blowing agent, catalyst and carbonizer. Therefore, in practical applications, it is necessary to consider not only the thermal decomposition of a single component and the matching degree of each other, but also the interaction between components.
3.2.2 Pyrolysis of intumescent fireproof coatings
Thermal analysis of LF solvent-shaped steel structure expanded fireproof coating was carried out, and the results are shown in FIG. 8.
LF solvent steel structure intumescent fireproof coating is a kind of intumescent fireproof coating with good fireproof performance, in which the flame retarder is ammonium polyphosphate, melamine and pentaerythritol. FIG. 8 shows the DSC curve of LF According to FIG. 1, FIG. 4 and FIG. 6, the action temperatures of the above three substances are 345℃, 290℃ and 360℃ respectively. As can be seen from Figure 8, heat absorption peaks of these substances appear respectively at these temperatures, indicating that the thermal properties of these substances do not change significantly after mixing. It is found that the matching of decomposition temperature of the carboniferous agent, blowing agent and carboniferous catalyst in the intumescent flame retardant has an important influence on the fire resistance of the fire retardant coating.
From the above experiments and analysis, it can be seen that the decomposition order of carbonizing agent, catalyst and blowing agent is more reasonable, and the decomposition temperature is not much different. Only the three match well, can achieve the best flame retardant effect. This can be used as a theoretical reference for the practical development and application of fire retarder.
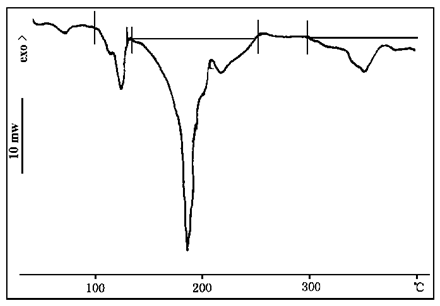
FIG. 4 DSC profile of ammonium polyphosphate
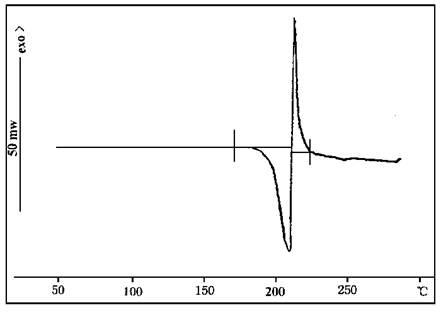
FIG. 5 DSC profile of ammonium dihydrogen phosphate
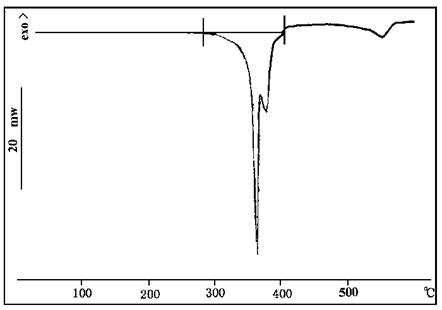
FIG. 6 DSC spectra of ammonium cyanide
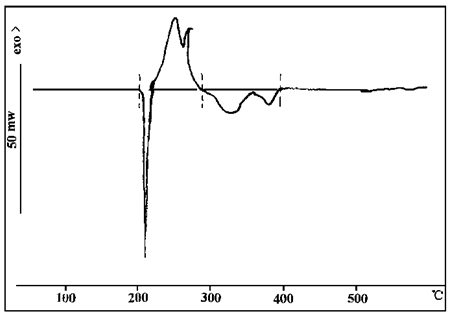
FIG. 7 DSC map of dicyandiamide
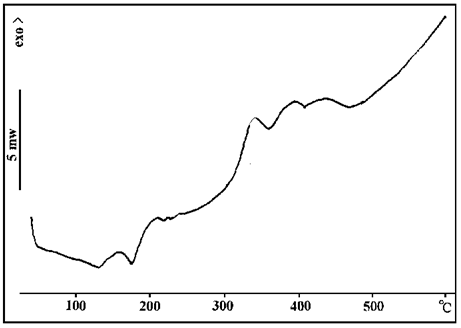
FIG. 8 DSC spectrum of LF solvent steel structure expanded fireproof coating
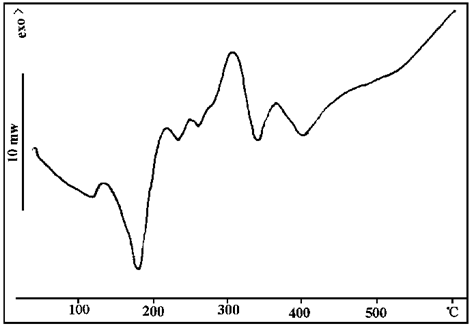
FIG. 9 DSC spectra of sample 3
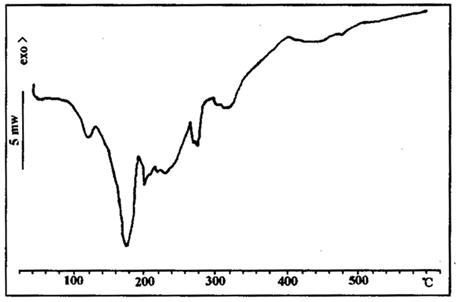
FIG. 10 DSC spectra of sample 2
4. Conclusion
4.1 Steel structure requires fireproof coating to work before 500℃, wood structure requires fireproof coating to work before 300℃, from the experiment, the decomposition temperature of commonly used flame retardant is lower than 500℃, and most of them are lower than 300℃. This meets the needs of practical applications.
4.2 The selection of the fire retardant formula is mainly based on the matching degree and action of the decomposition temperature of the char forming agent, blowing agent and catalyst, that is, whether a good char foam layer can be formed.
4.3 In the actual development and application of fire retardant, the mutual influence of the properties of each component is also an important factor in determining the performance of fire retardant, which should not be ignored.
References:
[1] Hu Yuan, Li Chun et al. Application of thermal analysis technology in the study of flame retardant materials [J]. Fire Science, 1999(1) : 74 ~ 77.
[2] Wang Yong, Li Jian. New flame retardants -- Synthesis and application of intumescent flame retardants [J]. Modern Fire and Product Research.1999(2).




