Domestic thermal analysis instrument professional manufacturer
Dedicated to thermal analysis instrument for more than 20 years
Application of DSC in starch
Differential scanning calorimetry (DSC) is a most commonly used thermal analysis technology, mainly used to directly measure the relationship between the physical properties of substances and temperature under the control of temperature program thermal analysis curve, especially suitable for studying the phenomenon accompanied by enthalpy change or specific heat capacity change. Starch, as the main storage of most higher plants, is composed of amylose and amylopectin, and many of its properties such as pasting, aging, glass transition and so on are related to heat, accompanied by changes in enthalpy or specific heat capacity. Therefore, many researchers have used DSC to detect the change of heat flow in the thermal phase transition process of starch, providing valuable reference for in-depth study of the structural changes of starch particles in the thermal phase transition process [1].
1 Differential Scanning-ning Calorimetry (DSC)
<section helvetica="" neue",="" "pingfang="" sc",="" "hiragino="" sans="" gb",="" "microsoft="" yahei="" ui",="" yahei",="" arial,="" sans-serif;="" letter-spacing:="" 0.544px;="" text-align:="" justify;="" text-indent:="" 2em;="" box-sizing:="" border-box="" !important;="" overflow-wrap:="" break-word="" !important;"="" style="-webkit-tap-highlight-color: rgba(255, 0, 0, 0); font-family: 微软雅黑, "Microsoft Yahei", Arial, Verdana, "sans-serif"; text-wrap-mode: wrap; margin: 0px; padding: 0px; max-width: 100%; color: rgb(51, 51, 51);">DSC (differential scanning calorimetry) [5] is a thermal analysis technique to measure the relationship between the energy difference delivered to the measured substance and the reference substance and the temperature under the program control of maintaining the same temperature of the sample and the reference substance. DSC has two sets of independent heating devices in the same temperature conditions using electrical compensation, and measure the sample's absorption of heat, the two heaters in the whole process to maintain a certain temperature range, you can accurately and quickly control the temperature and heat capacity, enthalpy measurement. DSC is divided into three types: power compensation type, heat flux DSC and heat flow type. It has the advantages of easy quantitative analysis, high resolution and high sensitivity. It can quantitatively determine a variety of thermodynamic and kinetic parameters, and can perform crystal microstructure analysis, such as the enthalpy change and specific heat capacity of the sample. The working principle of DSC is shown in Figure 16. The temperature difference between the sample and the reference object (ΔT) reflects the magnitude of the thermal effect. When DSC is operated, its sample size is very small, usually in the range of 10 to 20mg for solid samples and 10 to 20μL for liquid samples. The preparation and sampling of the sample have a great influence on the determination results.
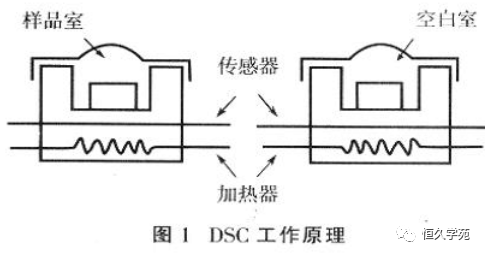
The temperature difference between the sample and the reference object (ΔT) reflects the magnitude of the thermal effect. When DSC is operated, its sample size is very small, usually in the range of 10 to 20 mg for solid samples and 10 to 20 μL for liquid samples. The preparation and sampling of the sample have a great influence on the determination results.
2 Gelatinization properties of starch
Starch and water mixed, starch particles will absorb water and expand, when heating starch milk, starch molecules began to shake violently, starch molecules and intermolecular hydrogen bond is interrupted, so in the original hydrogen bond position on the suction of a lot of water (hydration), starch crystallization zone began to slowly disappear, when the crystallization zone completely disappeared that is called gelatinization temperature, at this time for gelatinization temperature. Because the starch gelatinization process represents the transformation of starch molecules from an ordered state to a disordered state, and is accompanied by energy changes, it can be measured by DSC. Starch gelatinization is an important phenomenon in the process of food processing, such as baking of bread and cake, extrusion of cereals, etc., all depend on moderate starch paste. Scholars have always paid much attention to the technology of starch gelatinization, and studied starch gelatinization by different methods according to the properties of starch particles. These methods include: viscosity method, microscopic observation method, light transmission, birefringence method, etc., but these methods are limited by some factors such as starch/water ratio, temperature range, etc. DSC is not limited by these factors [8]. The reason is that A DSC can study starch gelatinization in a wide range of starch/ratio. A gelatinization temperature above 100℃ can be measured by DSC. A can estimate the enthalpy value of phase transition according to the test result of C [7].
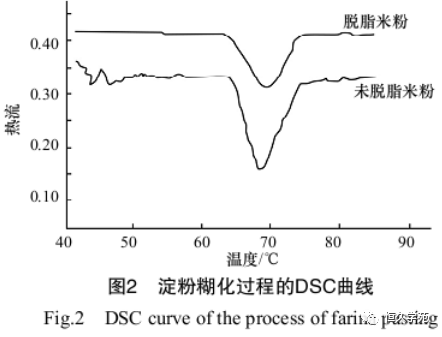
Liu Jingsheng et al. [9] used DSC to study the gelatinization process of starch in defatted and non-defatted rice flour, and the results are shown in Figure 2.
Aging of 3A starch
Starch gels can be obtained by cooling the gelatinized concentrated starch water suspension. In the process of gel aging, the rheological properties, crystallinity and water holding capacity change significantly, which is the process of starch aging. It is the main factor affecting the texture of starch food. It is generally believed that starch aging consists of two independent processes, (a) gelation of soluble amylose during gelatinization. (b) recrystallization of amylopectin within gelatinized starch particles. Quantitative DSC technology is usually used to study the rate and degree of recrystallization of amylopectin during starch aging. In other words,DSC technology is a feasible method to detect the formation process of recrystallized gel network. According to the size of the melting heat absorption peak in the DSC curve, the amount of aging starch crystals can be calculated, so as to judge the degree of aging of starch [10-11].
Ding Wenping et al. [12] used DSC to study the aging (rejuvenation) characteristics of glutinous wheat starch. The test condition was that the gelatinized sample was stored at 4℃ for 1, 3, 5, and 14 days, and then the rejuvenation was determined by DSC. The scanning range is 20 ~ 100 ℃, and the heating rate is 10 ℃/min. The results are shown in Table 4.
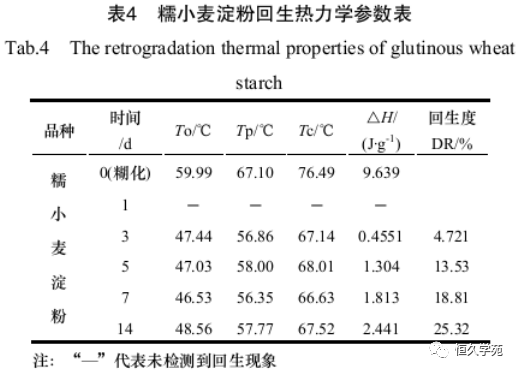
As can be seen from Table 4, gelatinized starch no longer has thermodynamic process during DSC analysis, and the aging degree varies with the different time of gelatinized starch placed at low temperature during cooling. The longer the time, the greater the degree of recovery, resulting in a large difference in the analysis results of starch after recovery, which is consistent with the theory of starch recovery. Therefore, the degree of starch recovery can be detected by DSC analysis.
4 Glass transition of starch
The glass transition is an important phase transition characteristic that affects the physical properties of macromolecular polymers. It is characteristic of amorphous polymers and is a second-order phase transition process [13]. At low temperatures, the molecules in the polymer's long chains are presented in a random manner.



