Domestic thermal analysis instrument professional manufacturer
Dedicated to thermal analysis instrument for more than 20 years
Application of thermal analysis technology in energetic materials

Industrial explosives need to experience a certain storage time from preparation to use, in this period of storage how to ensure the stability of explosives, which involves the stability of explosives. The stability of explosives refers to the ability to maintain its physical, chemical and explosive properties without significant changes under certain conditions. It is generally divided into physical stability and chemical stability. Physical stability refers to the ability of explosives to maintain their physical properties without significant changes under certain conditions. Chemical stability is the ability of an explosive to maintain its chemical properties without significant changes.
For condensed explosives, the thermal decomposition kinetics can be divided into the following three stages:
2) Decomposition acceleration period: After the end of the delay period, the decomposition speed gradually accelerates, and the speed can reach the maximum value at some point. This settlement is called the acceleration period:
However, when the amount of explosive is high, the reaction speed may also increase until the explosion. The division of the above stages is according to the properties of the dynamic curve, and does not involve the microscopic mechanism of explosive thermal decomposition. When the explosive molecule is decomposed, it is not immediately formed into the final product, but is segmented.
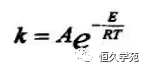
Where :k is the initial reaction velocity constant (1s) at a certain temperature; A refers to the pre-factor; T is the temperature; R is the universal gas constant (11kJKmol); E is the activation energy of the decomposition reaction (1kJmol).
The formula (1-2) is obtained by dividing (1-1)
1.2 Research methods of explosive thermal decomposition
Thermal Gravimetry (TG) is a method to determine the mass of substances as they change with temperature. It was first published in 1915 by Kotaro Honda of the University of Tokyo, Japan, who designed the first simple thermal balance. Due to the long measurement time and the limited stability of the instrument, the thermal balance has not been widely used. Then with the continuous improvement of thermal analysis technology. The above methods can only directly or indirectly measure the total amount of released gas and volatilization, as well as study the phenomenological reaction kinetics, but can not qualitatively and quantitatively measure the components and content in the gas, nor can they clarify the mechanism of the reaction. The Chemical Reactivity Test (CRT) developed on the basis of gas chromatography was first used to replace the vacuum stability test method in the Pantex factory in the United States in the early 1960s. This method can not only accurately determine the total amount of released gas, but also quickly determine the content of NO2,CO2, H2O,CO,NO and other components.
The chemiluminescence method developed in the early 1970s is mainly used to determine the content of NOx generated by the thermal decomposition reaction of explosives at lower temperatures, and its sensitivity can reach ppb level. It is of great significance for measuring the thermal stability and compatibility of explosives at storage temperature and predicting the storage life.
Temperature Modulation is introduced into thermal analysis, resulting in thermal analysis techniques such as MDSC and TMTG. They also play an important role in the thermal analysis process. In addition, the quasi-definite and quasi-isobaric TA techniques developed by Paulik et al. are promising thermal analysis methods.
The Accelerating Rate Calorimetry (ARC) test developed in recent years is a new method to evaluate the risk of exothermic chemical substances. The method is described in 2.1. Accelerated calorimeter is a new thermal analysis instrument recommended by the United Nations for the assessment of dangerous goods. The thermodynamic and kinetic information of exothermic reaction can be obtained by ARC test. The accelerated calorimeter can ensure that the sample in the test environment is completely adiabatic condition to measure the change of time, temperature and pressure during the sample thermal decomposition reaction. Various kinetic parameters of exothermic reaction can be determined by establishing mathematical model. According to these parameters, the risk of the reaction object can be accurately predicted. Compared with DSC and other methods, it has incomparable advantages, especially it can give the slow change process of pressure in the early stage of thermal decomposition of substances that can not be given by DTA and DSC.
Accelerated calorimeter is a calorimeter designed on the basis of adiabatic principle. It can provide time-temperature-pressure data of chemical reaction under adiabatic conditions with a large sample size and high sensitivity. It can accurately measure the initial temperature of sample thermal decomposition and the curve of temperature and pressure change with time during adiabatic decomposition. Various thermodynamic and kinetic parameters of exothermic reaction can be determined. According to these parameters, the risk of the reaction object can be accurately predicted.
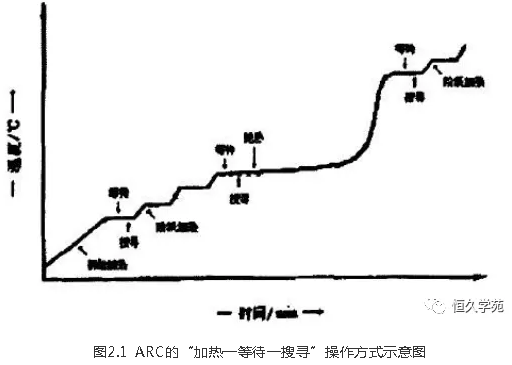
The acceleration calorimeter is started by setting the initial temperature, slope sensitivity, heating amplitude, termination temperature and waiting time on the ARC controller. The acceleration calorimeter quickly raises the temperature to the starting temperature and then automatically operates in a "heat-wait-search" manner (see Figure 2.1). In the "heating" stage, the temperature of the acceleration calorimeter rises according to the set heating range; In the "waiting" stage, the accelerated calorimeter establishes the temperature balance in the adiabatic furnace. ARC enters the "search" phase, comparing the temperature rise rate of the sample with the set slope sensitivity, if the former is less than the latter, it automatically enters the next "heat-wait-search" cycle; If the former is greater than the latter, the accelerated calorimeter automatically switches to the "exothermic" mode and records information such as temperature, pressure and temperature rise rate during the entire reaction process.
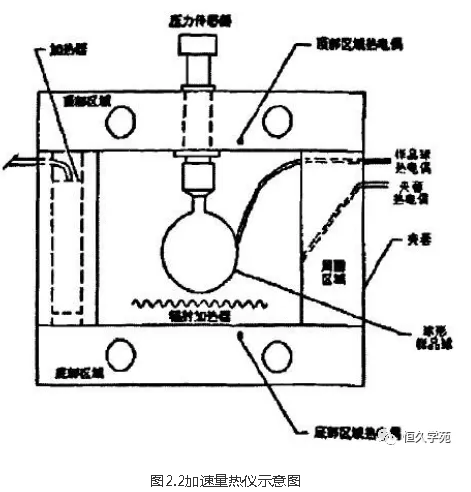
2.3 Structure of acceleration calorimeter
The core structure of CSI-ARC produced by Columbia Scientific Industries is shown in Figure 2.2. The spherical sample chamber (inner diameter 10mm) shown in the figure can accommodate solid or liquid samples of lOg. The body of the calorimeter is made of nickel-copper alloy, which is equipped with three thermocouples and eight heaters. They keep the temperature difference between the shell and the sample small throughout the operation. A fourth thermocouple is mounted on the outer wall of the sample chamber to detect the temperature of the sample, which is directly connected via a thin tube to a diaphragm pressure sensor that can continuously measure pressure changes.
2.4 Calculation and correction of dynamic parameters
Under adiabatic conditions, the general equation (*) of adiabatic temperature rise rate of acceleration calorimeter is
Where mt, dT/dt is the temperature rise rate of the reactant at temperature T; ΔTab is the adiabatic temperature rise of the reactant, ΔTab=Tf-To, where the sign is the temperature at the end of the reaction, and To is the initial heat release temperature of the inverse; f(a) is the reaction mechanism function, where
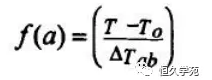
The kinetic data such as apparent activation energy Ea, pre-exponential factor A and reaction order can be obtained by ARC system calculation. However, the equation (*) is based on the ideal case that the heat released by the chemical reaction is used only to heat the specimen itself, without taking into account the presence of the reaction vessel. In fact, whether in practical applications or in ARC tests, part of the heat released by the reaction is used to heat the reaction vessel. Therefore, it needs to be amended. Correction factor introduced into the sample container:
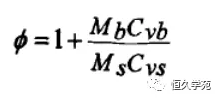
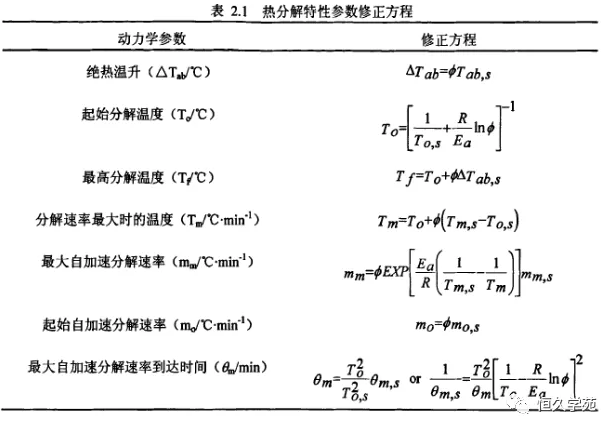
In the above formula, Ms is the sample size; Cvs, the average specific heat capacity; Is the mass of Mb sample container; Is the average specific heat capacity of Cvb sample container. The following table lists the modified equations for each parameter
3. Test results and analysis of accelerated calorimeter
ARC test results of emulsion explosive are shown in FIG. 3.1.
+
FIG. 3.1 Sample test curve
The test results of the sample are shown in Figure 3.1. The initial temperature of the test was 100℃. After 19 cycles of "heat-wait-search", the calorimeter detected heat release at 241.18℃, when the temperature rise rate was 0.049℃min-1 (greater than the slope sensitivity of 0.020℃min-1 set by the system), and then the temperature rise rate continued to decline. As can be seen from FIG. 3.1 (a), the discontinuity at 242.54 ~ 250.83℃ is similar to the discontinuity of nitric acid decomposition (both discontinuity ranges from 241.07 ~ 255.81℃), which is similar to the decomposition curve of SP-80/ nitric acid in a mixed system. Then, after going through the "heat-wait-search" process twice, the instrument began to record data again; This shows that sample 1# is accompanied by endothermic process during the decomposition process. From 250.83℃, the temperature rise rate increases slowly. After 235.65 min, the maximum temperature rise rate of 0.662℃min-1 appeared at 260.66℃, and then the temperature rise rate gradually decreased. The maximum decomposition temperature and pressure of the reaction system are 263.75℃, 265.321cPa and 6.851kPamin-1 respectively
4.Sum up
The ARC test results and test curves are given respectively. The adiabatic decomposition characteristic parameters are obtained, and a simple comparative analysis is made.
1) Compared with the research results, the influence of typical components of emulsion explosive on the thermal decomposition of emulsion explosive and ammonium nitrate is very similar, that is, the substances that promote the decomposition of ammonium nitrate are unfavorable to the thermal stability of emulsion explosive. Especially in the aspects of temperature rise rate and pressure change, the two laws are consistent: Because the emulsion explosive system is more complex, the decomposition curve becomes more complex;
2) In terms of initial decomposition temperature, maximum decomposition temperature and adiabatic temperature rise, the influence of each component on emulsified explosive and ammonium nitrate is different. The influence of continuous phase and oil phase components on ammonium nitrate is significant, but the influence on emulsion explosive is decreased, and the influence of emulsifier on emulsion explosive is obvious.
3) When the permitted emulsified explosive component of coal mine is changed to the above substances, its thermal stability decreases and decomposition accelerates.
Reference:
[1] Andreyev, trans. Stone Hair, Thermal decomposition of Explosives. Beijing: National Defense Industry Press [2] Lai Ying. Application prospect and development countermeasures of aqueous industrial explosives. China Coal, 1999 [3] Li Jianjun, Wang Guangxu. Experimental study on hot spot ignition of emulsion explosive. Engineering Blasting, 1997.6 [4] Zhou Xinli, Liu Zuliang, Lu Chunxu. Analysis of thermal decomposition safety of rock emulsion explosive by accelerated calorimetry. [J]., 2005, 26 (2):62 ~ 65 [5] Chen Jian-Ping, Wang Jin, Calculation of chemical reaction kinetic parameters of emulsified explosives. Coal, 2000.4 [6] Wang Guanglong, Xu Xiucheng. Study on thermal stability of nitric acid. Journal of Zhengzhou University, 2003, 24 [7] Lu Ming. Formula design of industrial explosive. Beijing: Beijing Institute of Technology Press, 2002 [8] Editorial Committee of the Chemical Fertilizer Industry Corpus. Chemical fertilizer industry. Beijing: Chemical Industry Press.



